BlueWillow’s NanoVax® adjuvant and antigen delivery platform is a first-in-class and best-in-class solution for intranasal vaccine development. The platform has established a strong human safety record with multiple clinical trials, and broad immunogenicity as demonstrated clinically and preclinically in numerous studies across a wide range of viral and bacterial pathogens.
Originally invented at the University of Michigan, the NanoVax® platform creates vaccines that elicit a three-pronged mucosal, cellular, and systemic immune response needed for robust protection against a range of infectious diseases and food allergies.
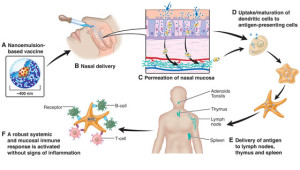
NanoVax® utilizes an oil-in-water emulsion of relatively large particle size of approximately 400nm. The oil droplets enable permeation of the nasal mucosa but prohibit crossing of the blood brain barrier. Compatible with a wide array of antigens and with inherent antiviral action, the technology efficiently delivers antigens directly to antigen-presenting cells for uptake but also adjuvants the immune response.
NanoVax® elicits a Th1 (non-inflammatory) immune response biased over Th2 (inflammatory), which is ideal for protection against mucosal respiratory and sexually transmitted pathogens. T-cell and B-cell homing to the mucosa is triggered for durable cell-mediated immune memory and elicits IgG and IgA immune responses. In our recent H5 clinical trial, strong immune system priming and systemic immune signals were demonstrated in previously pathogen-exposure naive subjects. The systemic response has not been demonstrated for previous intranasal flu vaccine candidates.
The limitations of non-adjuvanted approaches to intranasal vaccination are well-known. There is now an adjuvant available that can be used safely in humans intranasally to enable the long sought-after next generation of vaccines.
Intranasal Adjuvanted Vaccine Development
There are many advantages of delivering vaccines via a simple nasal spray vs. an injection through the skin:
- Needle-free vaccine delivery overcomes a significant portion of vaccine reluctance amongst the public. Self-administration is enabled.
- The nature of the immune response (balanced mucosal, cellular and systemic immunity) mirrors that generated by natural infection in contrast to vaccinating intramuscularly against pathogens that infect via the airway.
- Supply chain costs are reduced addressing barriers to vaccine in low- and middle- income countries and regions.
How Do Intranasal NanoVax® Vaccines Work?
Our best-in-class NanoVax® adjuvanted vaccines are applied intranasally via a simple spray device. As shown in the figure below, the adjuvant efficiently delivers antigens to local dendritic cells. The dendritic cells take-up and deliver the antigen(s) to regional lymph nodes initiating the immune response. This path of vaccination results in potent mucosal, systemic and cell-mediated immunity.
Human Clinical Evidence
The NanoVax® intranasal platform has demonstrated safety in humans in three completed Phase 1 clinical trials. The most recent Phase 1 study in H5 influenza, completed in 2025, demonstrated strong pathogen-specific mucosal and humoral immunity as measured with a broad array of relevant markers. The study results are available in preprint (link below), have been peer-reviewed and accepted for publication which is anticipated in early Oct. 2025.
News
- BlueWillow Biologics’ Intranasal Bird Flu Vaccine Shows Signs of Broad Immune Response in Phase I Clinical Trial Published in Nature Communications
- H5 Influenza (Pandemic Flu) Clinical Trial Successful, Peer-Reivewed and Accepted for Publication
- BlueWillow Biologics to Present Significant Progress on Its Novel Intranasal Vaccine Technology at World Vaccine Congress Europe
Contact
BlueWillow Biologics, Inc.
2311 Green Rd. Suite A
Ann Arbor, MI 48105
734-302-4000
734-302-9150 fax

